From the JNCI: Journal of the National Cancer Institute
We sought to determine the strength of the evidence suggesting that estrogen and postmenopausal replacement hormones play a role in the development of breast cancer. We reviewed the existing English language literature in MEDLINE® on hormones and breast cancer, including reports on cell proliferation and endogenous hormone levels, as well as epidemiologic studies of the relationship between the use of postmenopausal hormones and the risk of breast cancer in women. A factor that increases the probability that cancer will develop in an individual has been defined as a cancer cause. The Hill criteria for demonstrating a link between environmental factors and disease were used to review the evidence for a causal relationship between female hormones and breast cancer. We found evidence of a causal relationship between these hormones and breast cancer, based on the following criteria: consistency, dose-response pattern, biologic plausibility, temporality, strength of association, and coherence. The magnitude of the increase in breast cancer risk per year of hormone use is comparable to that associated with delaying menopause by a year. The positive relationship between endogenous hormone levels in postmenopausal women and risk of breast cancer supports a biologic mechanism for the relationship between use of hormones and increased risk of this disease. The finding that the increase in risk of breast cancer associated with increasing duration of hormone use does not vary substantially across studies offers further evidence for a causal relationship. We conclude that existing evidence supports a causal relationship between use of estrogens and progestins, levels of endogenous estrogens, and breast cancer incidence in postmenopausal women. Hormones may act to promote the late stages of carcinogenesis among postmenopausal women and to facilitate the proliferation of malignant cells. Strategies that do not cause breast cancer are urgently needed for the relief of menopausal symptoms and the long-term prevention of osteoporosis and heart disease. [J Natl Cancer Inst 1998;90:814–23]
During the past 40 years, we have moved from a period when the use of postmenopausal estrogen was advocated to reduce the risk of breast cancer to the present, when a plethora of epidemiologic studies suggests a positive relationship between estrogen levels, markers of estrogen exposure, the use of estrogens after menopause, and an increased risk of breast cancer. Numer
ous meta-analyses of the published studies concerning the use of postmenopausal hormones provide a quantitative summary of the field, indicating that a significant relationship exists between the duration of use of postmenopausal hormones and the risk of breast cancer (1,2). Furthermore, the combined reanalysis of original data from 51 epidemiologic studies that included more than 52 000 patients with breast cancer and more than 100 000 women without breast cancer indicates that, for each year a woman uses postmenopausal hormones, her risk of breast cancer increases by 2.3% (95% confidence interval [CI] 1.1%-3.6%; P .0002), and the findings do not vary significantly between studies (3). The remaining uncertainty relates in large part to how varying formulations of hormones affect the magnitude of this relationship, rather than to the question of whether or not the relationship is present.
Although early meta-analyses examined the association between ever use of postmenopausal hormones and risk of breast cancer (4), this is no longer the clinically relevant issue. The vast majority of hormone use at that time was short term, for relief of menopausal symptoms. Long-term use of postmenopausal hormones is now established to reduce the risk of coronary heart disease, osteoporotic hip fractures, and other illnesses. The clinical question therefore has become, “ Does long-term use of hormone replacement increase risk of breast cancer and, if so, how soon after use has begun does risk increase?”
In this review, I summarize the evidence that endogenous estrogen and replacement hormones are causally related to breast cancer. In discussing replacement hormones, I consider the methodologic biases in many of the epidemiologic studies published to date that have resulted in our underestimating the adverse effect of postmenopausal hormones. For this review, all epidemiologic studies are considered, and major emphasis is placed on results from systematic reviews and meta-analyses that address pertinent issues, such as the variation in results among studies. First, I will review the biology that relates hormones to breast cancer. Studies were identified by searching MEDLINE® for all reports in the English language relating estrogen to mammography and breast cancer in women.
Reproductive Events and Risk of Breast Cancer
Since the 1970s, strong evidence has been in place to show that early menopause is associated with a reduced risk of breast cancer (5–8). For every 1-year increase in age at menopause, the risk of breast cancer increases by approximately 3% (9,10). The early studies were conducted in a time before the widespread use of postmenopausal hormones and, therefore, are not biased by variation in the pattern of use according to the type of menopause and the age at menopause. These early results have been replicated in more recent studies (3,9).
Additional reproductive factors that influence hormonal exposure of the breast and breast cell proliferation include age at first pregnancy, number of children, and age at menarche (9–11). Evidence suggests that the first pregnancy, but not subsequent pregnancies, is related to a one-time increase in the risk of breast cancer (12) that results in higher rates of breast cancer among parous as compared with nulliparous women through the early reproductive years (13). This cross-over of parous women experiencing higher rates of breast cancer than nulliparous women has been described in numerous settings and by use of a wide range of study and analytic approaches. Later age at first birth is associated with a greater adverse effect of the first pregnancy (9).
The duration from menarche to menopause summarizes the traditional lifetime exposure of women to significant levels of reproductive hormones. After menopause, hormone levels are substantially lower. During the premenopausal period, the timing of pregnancies conveys risk; late pregnancies are associated with increased risk of breast cancer, and early pregnancies are protective, presumably because first pregnancy is associated with terminal differentiation of breast cells and the cell cycle is longer after first pregnancy, allowing more time for DNA repair (14). Furthermore, the traditional reproductive patterns experienced in the developing world (where six or more pregnancies are the average reproductive rate) (15) convey substantial protection against breast cancer. Comparing the reproductive pattern with six or more pregnancies with the typical pattern of two pregnancies in the developed world, we have shown that at least 50% of the international variation in breast cancer rates can be explained by these patterns of childbearing (16).
In summary, reproductive events have a substantial impact on a woman’ss lifetime risk of breast cancer. Although they are markers of exposure to endogenous reproductive hormones, these events are now thought to influence the rate of breast cell growth and the accumulation of DNA damage.
Hormonal Exposure After Menopause
Obesity is positively related to reproductive hormone levels among postmenopausal women (17–19). This finding reflects the biologic function of fat cells, which metabolize androgens to estrogens among postmenopausal women. For example, the mean estradiol concentration among a sample of women from the Nurses’s Health Study was 4.7 pgamong women with a body mass index less than 21 kg2 and 10.0 pgamong women with a body mass index greater than or equal to 29 kg2(18).Lean, postmenopausal women have both lower estrogen levels and lower age-specific incidence of postmenopausal breast cancer (20–22).
Obesity is also positively related to risk of breast cancer mortality (23,24). This relationship persists after control for stage at diagnosis and other modifiers of survival (24). This is consistent with a role for estrogen as a late promoter of breast cancer. Tamoxifen, which acts as an antiestrogen in the breast, significantly reduces the risk of mortality among women diagnosed with breast cancer (25). Further evidence for the role of estrogen in facilitating the growth of tumors among women already diagnosed with breast cancer is the long tradition of using oophorectomy as a successful approach to the treatment of breast cancer (25). Ovarian ablation significantly reduces mortality— by 26% among women under age 50 years at diagnosis of breast cancer.
Taken together, these data indicate that higher levels of estrogens among postmenopausal women are associated with increased incidence of breast cancer and that higher levels after diagnosis lead to poorer survival.
Breast Cancer Risk and Markers of Hormonal Exposure
Several studies have explored bone density in relation to risk of breast cancer. Low bone density reflects lower levels of estrogen (26,27). The prospective study of osteoporotic fractures (28) indicated that bone density in the wrist, hip, and spine has a strong and statistically significant direct relationship with the risk of breast cancer. Also, data from the Framingham Study (29) showed that bone density as assessed by uniform review of hand x rays is directly related to the risk of breast cancer. The relative risk (RR) in this study between extreme quartiles of bone density in the population was 3.5 (95% CI 1.8–6.8) and was comparable to the results for hip and spine reported from the study of osteoporotic fractures (28). These data on bone mass support previous results from studies of women with fractures (30,31)indicating that high bone mass is a marker for estrogen exposure and risk of breast cancer.
These data indicate that bone density, used as a marker of postmenopausal estrogen exposure (and perhaps longer exposure), is directly related to the risk of breast cancer. This adds support to the biologic role of estrogen in the etiology of breast cancer.
Mammographic density also provides insight into breast cancer risk— denser breasts are associated with higher breast cancer incidence (32,33). A number of studies (34–37) indicated that, after commencing use of replacement hormones, there is a significant increase in breast density and that use of hormone replacement inhibits the involutional process in the breast following menopause.
Hormone Levels and Breast Cancer Risk
Few studies have assessed prospectively the risk of breast cancer according to the levels of hormones in the blood. The results from such studies have varied, perhaps because of the difficulty in measuring hormone levels in postmenopausal women (38). In a follow-up study that identified 130 women diagnosed with incident breast cancer, Toniolo et al. (39) showed a positive relationship between serum estradiol levels and breast cancer. The follow-up of the study of osteoporotic fractures (40)also showed a strong relationship between hor-
mone levels among women who were not taking postmenopausal hormones and their subsequent risk of breast cancer. Comparing extreme quartiles of estrone levels, Cauley et al. (40) observed an RR of 3.2 (95% CI 1.4–7.0) for women in the highest quartile. Similar data were observed among women on the island of Guernsey, where 61 women were diagnosed with breast cancer (41). Baseline blood levels of hormones showed a higher mean among Guernsey women subsequently diagnosed with breast cancer than among those who remained free from breast cancer. Other prospective studies agree with this relationship, although the number of cases is small (42–44), or have failed to find such a statistical association but are compatible with the presence of a statistically significant relationship between increasing risk and higher hormone levels (45,46).
The data from prospective studies have been combined in a systematic review and quantitative analysis by Thomas et al. (47), who noted that there was no substantial heterogeneity in results among the prospective studies. These authors observed a statistically significant higher risk of breast cancer among women with higher levels of serum estradiol.
Androgens have also been reported to be associated with an increased risk of breast cancer in some (43,45) but not in all (46,48) prospective studies addressing this relationship. However, in two of the larger prospective studies (41,48), the findings were mixed. Although the data in the study by Zeleniuch-Jacquotte et al. (48) that included 81 cases showed a statistically significant positive relationship between testosterone levels and risk when adjusted only for age— once other hormones such as estradiol and percent sex hormone-binding globulin-bound estradiol are controlled for— the results showed no statistically significant dose-response relationship, and the RR, comparing highest versus lowest quartile of serum testosterone, was not significant (RR 1.2; 95% CI 0.4–3.5). In a smaller study that involved only 24 cases, Berrino et al. (43) did not see a comparable effect when they controlled for total estradiol. Thomas et al. (41), on the other hand, who had 61 cases in their prospective study, observed that the statistically significant positive relationship between testosterone levels and the risk of breast cancer became null after control for estradiol levels. Taken together, these data suggest that testosterone may have an indirect effect through estrogens rather than being an independent risk factor. Additional data are, however, required to confirm this hypothesis.
In conclusion, growing evidence supports a positive relationship between blood levels of estrogens and the risk of breast cancer, with up to a fourfold increase in risk between extreme quartiles of estradiol. This association is stronger than that between cholesterol levels and coronary heart disease, suggesting that estrogen levels may serve as a clinical indicator of breast cancer risk once laboratory methods to measure estrogens are adequately standardized and once the best estrogen fraction to predict risk is identified (38).
Correlates of Use of Postmenopausal Hormones
Traditionally, the use of postmenopausal hormones has been for relief of menopausal symptoms. More recently, their use has been advocated to reduce the risk of coronary heart disease and osteoporosis (49). Mounting evidence suggests that women with
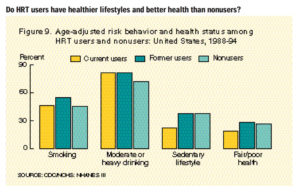
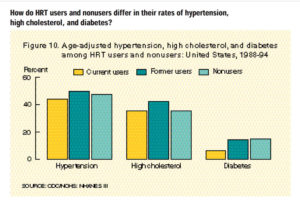
menopausal symptoms have lower estrogen levels at menopause than women without symptoms (50–53). Women who are lean at menopause are more likely to report hot flashes (50) and to use postmenopausal hormones (54). In addition, bone density is lower in women when they begin taking postmenopausal hormones than in those who choose not to take them (55). Together, this evidence points to the likelihood that women who take postmenopausal hormones are, on average, at lower risk of breast cancer at the time of menopause than women who do not take hormones. This situation would lead to an underestimate of the adverse effects of postmenopausal hormones.
In addition to these biologic correlates of use of hormones, women who take hormones have been noted to be of higher socioeconomic status and to consume more alcohol (56,57) — factors that are associated with increased risk of breast cancer. Likewise, women who take postmenopausal hormones may have higher rates of mammography, which would lead to a higher reported incidence of breast cancer. These factors would lead to overestimating the underlying biologic relationship between use of hormones and risk of breast cancer (58); however, they can be controlled in analysis.
Only some of these apparent sources of bias can be controlled during data analysis— many are not measured in the majority of epidemiologic studies of hormone use and the risk of breast cancer. Therefore, it remains possible that there is confounding by indication in these studies: The unmeasured correlates of hormone use result in women at lower risk of breast cancer being more likely to take postmenopausal hormones. Such confounding by indication would lead to a biased estimate of the relationship between use of hormones and risk of breast cancer.
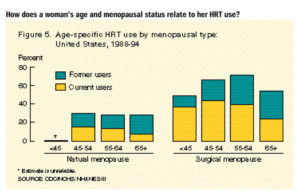
In addition, women who undergo menopause at an earlier age are more likely to take postmenopausal hormones (59) and to take them for longer durations than women who undergo surgical menopause (60). Because early menopause is associated with a substantially lower risk of breast cancer (5,6), users of hormones again are, on average, at lower risk of breast cancer than nonusers of the same age, once other risk factors are controlled for. Unpublished data from the Nurses’s Health Study illustrate this relationship. Among women 55–59 years old who had menopause before age 47, there is substantial variation in the duration of menopause depending on the category of use of postmenopausal hormones, making this a potential confounding factor. For never users of hormones, the mean time since menopause is 15.2 years; for current users of less than 5 years’s duration, it is 16.1 years; and for current users with 5 or more years of use, it is 16.6 years. The variation in time since menopause is more extreme in younger age groups. Clearly, in analyses controlling for age at menopause in three categories (<47 years, 47–52 years, and =52 years) as in previous analyses (61), we have not controlled for age at menopause with sufficient rigor to remove bias in our estimates. Thus, this approach to statistical control does not give an unbiased estimate of the relationship. Further illustration of this bias is seen in the collaborative reanalysis of epidemiologic studies (3). When the Oxford investigators (3) assessed the potential magnitude of risk underestimation that can be attributed to incomplete analytic control for the time since menopause in previous reports of epidemiologic data, they found a profound impact, an underestimation of the strength of the association. They noted that the estimated annual
increase in the risk of breast cancer associated with the duration of use of replacement hormones was 0.8% per year (P .10) if no correction was made, compared with an increase in risk of 2.3% per year (P .0002) when full statistical control was implemented.
In summary, the adverse effects of postmenopausal hormones will likely have been underestimated in individual epidemiologic studies that compare hormone users with nonusers. The majority of studies of hormone use do not use measures of bone density or menopausal symptoms as markers of hormone levels, and few studies have collected blood samples to assess prospectively the underlying levels of circulating hormones. Substantial residual confounding in numerous epidemiologic studies published to date, such that the women taking hormones for longer durations will have had earlier menopause (and so be at lower underlying risk of breast cancer) than the women taking hormones for shorter durations, may have led researchers and clinicians to underestimate the adverse effect of hormones on breast cancer risk.
Increasing Risk of Breast Cancer With Longer Duration of Use of Postmenopausal Hormones
Use of postmenopausal hormones increases the level of estrogens in postmenopausal women. Both oral estrogen replacement and transdermal estrogen raise serum estradiol levels to those found in premenopausal women who are in the early follicular phase of the menstrual cycle (62,63). A positive relationship between the duration of use of hormones and the risk of breast cancer has been reported by many investigators who have conducted meta-analyses of the published epidemiologic studies (1,64–66). Steinberg et al. (2) conducted an extensive, systematic review of the evidence. They noted that, among the U.S. case-control studies published from 1977 to 1991, a positive relationship was observed between the duration of hormone use and the risk of breast cancer in 11 of 12 studies with community control subjects and in four of nine studies with hospital-based control subjects. Among the prospective studies, an increased risk with increasing duration of use was reported in all four studies. The focus here was not on the relationships attaining statistical significance, but rather on the direction of the relationship between use of postmenopausal hormones and risk of breast cancer.
Since that review, additional studies (59,61,67,68) have been published. Although they do not uniformly support the relationship between duration of use of postmenopausal hormones and breast cancer risk, they do not rule it out. Furthermore, these studies all use broad categories of age at menopause when they control for this important predictor of risk. Case-control studies have been less consistent in observing the relationship between duration of use and breast cancer risk. This is evidenced by greater levels of among-study variation or heterogeneity in the results as reported by Steinberg et al. (2). One reason for this may be the potential for bias through differential participation by control subjects who do or do not take hormones. We might assume that hormone users are more health conscious and, therefore, more likely to participate in health-related studies. Such overrepresentation of hormone users among control subjects would bias the relationship between hormones and breast cancer toward the null result.
With collaborative input from investigators responsible for the vast majority of all studies to date of exogenous hormones and breast cancer risk, investigators at Oxford University (3) undertook a combined reanalysis of original data from 51 epidemiologic studies that included more than 52 000 women diagnosed with breast cancer and more than 100 000 women without breast cancer. Using consistent analytic methods across all studies and controlling established risk factors for breast cancer, these data indicate that, for each year of use of postmenopausal hormones, the risk of breast cancer increases by 2.3% (95% CI 1.1%-3.6%; P .0002) and that the findings do not vary significantly between studies (3). The major hormone used in these studies was estrogen without progestins (unopposed estrogen). For women who were current users and had taken hormones for 5 years or longer, the RR was 1.35 (95% CI 1.21–1.49) compared with never users. On the basis of these data, it is estimated that, for every 1000 women who begin to take postmenopausal hormones at age 50 and who take them for 10 years, there are six excess cases of breast cancer; for 15 years’s duration of use, there are 12 excess cases.
The combined epidemiologic data, therefore, now show a significant increase in the risk of breast cancer with increased duration of use of postmenopausal hormones. The estimates from epidemiologic studies may, however, still underestimate the magnitude of this association because of incomplete control for predictors of use of hormones and for the underlying hormone levels.
Mechanisms by Which Hormones Can Influence Breast Cancer Risk
Much evidence now supports the concept of cell proliferation as the underlying process by which DNA damage (genetic errors) accumulates and the risk of breast cancer increases (69,70). Among premenopausal women, the proliferative activity of the breast is greatest during the luteal phase of the menstrual cycle (71,72).Progesterone may therefore be the major mitogen in the normal breast epithelium of premenopausal women (73). Further evidence in support of this mechanism for progesterone comes from a study of surgically postmenopausal Cynomolgusmacaques (74) that were treated for 30 months with either conjugated equine estrogen or a combined therapy of conjugated equine estrogen plus medroxyprogesterone acetate. In that study, the combined therapy induced greater breast cell proliferation than did conjugated equine estrogen alone. In contrast to this in vivo study, the proliferation of breast cells in vitro has mostly been stimulated by estrogens and inhibited by progestins [reviewed in (75) ]. Thus, uncertainty persists as to the role of progestins and whether the pattern of use (continuous versus interrupted) may also modify the risk of accumulating DNA damage and of progression to breast cancer.
In the postmenopausal period, cell proliferation is very low (76). Pike et al. (70)estimated that the use of unopposed estrogen after menopause should raise the annual rate of increase in the risk of breast cancer by 2.1% above that observed among women not taking hormones. They also predicted that the addition of progestins will further increase the rates of cell proliferation and of breast cancer. Evidence from studies of the change in mammographic patterns with alternative regimens of
replacement hormones offers some support for this proposed relationship. Women treated with combination estrogen plus progestins showed a greater increase in mammographic density than did women who took estrogens alone (34,35).
Evidence from studies of hormonal carcinogenesis suggests that estrogens may have dual effects, causing both nongenotoxic cell proliferation and genotoxic effects [reviewed in (77) ]. Evidence from epidemiologic studies shows a stronger relationship between the current use of replacement hormones (typically unopposed estrogen) and the risk of breast cancer than is seen for former use of these hormones. This suggests that the major effect of the replacement hormones may be through the promotion of cancer growth rather than through genotoxic effects. In the combined reanalysis of epidemiologic studies (3), those who had stopped taking hormones more than 5 years previously were not at increased risk compared with never users, regardless of how long the women had taken replacement hormones. Such women had, however, probably taken estrogen alone; the contribution of estrogen plus progestins to breast cancer risk among former users has not been addressed because this regimen has come into use only recently.
Increase in Breast Cancer Risk by Use of Estrogen Alone or Estrogen Plus Progestin
Most epidemiologic studies linking postmenopausal hormones to risk of breast cancer in the United States have focused on the use of unopposed estrogen. Recently, the addition of progestins to the regimen has become more widespread to counter the adverse effects of estrogen on the risk of developing endometrial cancer. Gambrell et al. (78) reported that the use of estrogen plus progestin is associated with a reduced risk of breast cancer. Although mechanisms have been proposed to account for this (e.g., sustained progestin levels may reduce the rate of cell division), the accumulating epidemiologic evidence does not support the view that combination therapy reduces breast cancer risk (3).
At the cellular level, as noted above, the division of breast cells is stimulated by progestins (70). We (61) and other investigators (79) have reported results indicating that estrogen plus progestin is associated with a higher risk of breast cancer than is estrogen alone. Other studies are compatible with an increase in risk among women taking estrogen plus progestin. It is of note that the Oxford University study (3)combined reanalysis of epidemiologic studies did not find evidence to support a lower risk among women taking combination therapy compared with women taking estrogen alone. Rather, in that combined analysis, although the number of breast cancer cases was relatively small among women whose duration of current use was greater than 5 years (58 cases), treatment with estrogen plus progestin was associated with a higher risk of breast cancer (RR 1.53; 95% CI 0.80–2.92) in these women than was the use of unopposed estrogens (RR 1.34; 95% CI 1.12–1.59); this difference was not statistically significant. Furthermore, there was statistically significant heterogeneity in the results for current use of 5 or more years’s duration according to the types of hormones used (3). Among women with shorter durations of use and among past users, there was no such heterogeneity evident for type of hormone. It is, therefore, more likely than not that adding progestins
to postmenopausal estrogen therapy will cause an increase in cell division and, with this, a greater accumulation of DNA errors that lead to breast cancer or to a greater proliferation of malignant cells.
Questions do remain, however, with regard to the potential for sustained progestin levels to move cells into a phase of reduced division— for arrest in early G1(75). To date, the epidemiologic evidence is not sufficient to address this question, in large part because the upswing in progestin use is recent and, hence, there is a lack of data from women who have experienced sustained or continuous long-term use of progestins.
Longer Survival Among Women Diagnosed With Breast Cancer While Taking Postmenopausal Hormones Than Among Other Breast Cancer Patients
Estrogen may induce a less malignant form of endometrial cancer in women who are taking postmenopausal hormones. The question has been asked as to whether the same may apply for breast cancer. Studies of mortality in women diagnosed with breast cancer (80,81) suggested that, when the survival of users is compared with that of nonusers, the users have an improved survival. The study by Bergkvist et al. (80) could not address the stage at diagnosis; however, when Strickland et al. (81)controlled for stage at diagnosis, the apparent survival advantage for prior use of estrogen was eliminated.
Evidence from the American Cancer Society Cancer Prevention Study II (82) also addresses this issue. In that prospective study, breast cancer mortality during 9 years of follow-up was lower among women who had taken estrogens after menopause than among women who never had taken estrogens. This result may again reflect better survival among women diagnosed while taking postmenopausal hormones, or it may reflect the lower underlying risk of breast cancer among women who have taken hormones than among those who have not. In the American Cancer Society study, 43.7% of the women who took hormones did so because of menopausal symptoms, 39.5% took hormones after a hysterectomy, and 7% took hormones for other reasons (82).
Mechanisms for the observed apparent improvement in survival among women who were taking postmenopausal hormones at the time of diagnosis could include a higher probability among these women of having estrogen-sensitive tumors. If this is true, then these women may be more likely to respond to antiestrogen therapy and, therefore, have a higher probability of survival after diagnosis than the average woman who is not taking hormones. On the other hand, the withdrawal of the exogenous estrogen after diagnosis may lead to better survival among women with such estrogen-responsive tumors. Alternatively, supplemental estrogen may promote slower growing tumors or discourage the development of more aggressive tumors, or it may be associated with more intensive screening (83). These issues remain to be resolved through further study.
Criteria for a Causal Relationship
A cause of cancer is a factor that increases the probability that cancer will develop in an individual (84). Hill (85) proposed a range of items that should be considered when reviewing scientific evidence to assess whether a causal relationship exists. Although not all of these criteria need to be met (86), the evidence regarding postmenopausal hormone use and breast cancer is now clearly consistent with a causal relationship. Below, I summarize the evidence according to the Hill criteria— with the exception of analogy, which has little to do with deductive logic (87).
Strength of Association
Consistent with the estimates developed by Pike and colleagues (70) on the basis of hormone levels observed among postmenopausal women, the annual increase in the risk of breast cancer after menopause among women taking estrogen alone is approximately 2% per year. The effect of adding postmenopausal hormones is comparable to that of delaying menopause (3). Thus, the association, although not strong by epidemiologic standards, is of the magnitude predicted by the underlying biology. Among current users with 5 or more years of use, the RR was 1.35 (95% CI 1.21–1.49) compared with never users.
Consistent Relationship
Absence of heterogeneity in results may be used as an indication of consistency (88).The increase in the risk of breast cancer with increasing duration of use of postmenopausal hormones is reported consistently and, when the combined reanalysis examined for heterogeneity in results among studies, no substantial or statistically significant heterogeneity was observed.
Dose-Response Relationship
Evidence comes from two areas to address the issue of a dose-response relationship between postmenopausal hormones and breast cancer. First, the blood level of estrogen relates directly to the risk of breast cancer in a dose-response pattern. This pattern was observed in the majority of prospective studies and was substantiated in the combined analysis by Thomas et al. (47). Second, the relationship between increased duration of use of replacement hormones and increased risk reflects a dose- response relationship in which duration of use represents a time-integrated dose (3).
Biologic Mechanism
The presence of estrogen receptors on breast cells, the action of the antiestrogen tamoxifen in reducing mortality after diagnosis in postmenopausal women, and the relationship between estrogen, progestins, and cell division, all point to a role for estrogen in breast cell growth and division. The presence of higher risk breast mammographic patterns among women who are treated with replacement hormones is further evidence that cell proliferation occurs in response to exogenous hormones.
Specificity
The increase in the risk of cancer among women who take postmenopausal hormones is not limited to breast cancer; it is also observed for endometrial cancer, for which a strong body of evidence supports a hormonal basis for the disease. Thus, like other chronic diseases, the requirement of specificity is not a central consideration for a causal relationship.
Relationship in Time
Evidence from prospective studies most clearly shows a relationship between current use of hormones and increased risk of breast cancer. Although the theory linking cell proliferation and increased risk of cancer would support an increase in risk regardless of current or past use, the epidemiologic data suggest that current use is associated with the proliferation of cancer cells and that the risk remains elevated 5 or more years after the use of replacement hormones has stopped. Studies based on blood levels of estrogen and bone density both indicate that estrogen status among postmenopausal women predicts their future risk of breast cancer. The ability to make an earlier prediction of increased risk on the basis of blood levels of estrogen is no doubt due to strong tracking (high correlation over time) of hormone levels among postmenopausal women (89). In the majority of studies, there is little doubt that the exposure of interest (estrogen) precedes the diagnosis of cancer. Perhaps more importantly, the data on estrogen status may support a role for postmenopausal estrogen exposure as a promoter of the progression and proliferation of breast cancer, not as an initiator.
Experiment
Clinical trials in which patients are randomly assigned to treatments are considered to be the best strategy to assess the risks and benefits of hormone replacement therapy by eliminating bias (90). To date, no substantial randomized trial has been completed that was designed to quantify the relationship between use of postmenopausal hormones and the risk of breast cancer. Such evidence is expected to be forthcoming from the Women’ss Health Initiative. This randomized design may reduce any bias (including confounding by indication) in epidemiologic studies that would lead to the underascertainment of breast cancer risk.
Coherence of the Evidence
The evidence reviewed above shows a relationship between endogenous hormone levels and the risk of breast cancer. Furthermore, there is a positive relationship between the long-term use of postmenopausal hormones and an increased risk of breast cancer. The strong protective effect of early menopause and the relationship between postmenopausal obesity and estrogen levels combine to support the role of estrogens in the etiology of breast cancer.
In summary, the causal relationship between endogenous estrogens, hormone replacement therapy, and breast cancer is supported by consistency, a dose-response relationship, biologic plausibility, temporality, strength of association, and coherence. Experimentation and specificity conditions are not met, as is seen in a review of recent cancer epidemiology (86). The use of consistency, strength, biologic plausibility, and dose-response information is compatible with the fact that these are the most commonly used criteria in drawing a causal inference from epidemiologic data.
Implications for Women Who Take Postmenopausal Hormones
Based on this review, it is evident that postmenopausal hormones cause breast cancer. Long-term use substantially in-creases the risk of this cancer, which is the most common cancer in women. Given this causal relationship and consistent evidence that hormone replacement reduces the risk of cardiovascular disease, osteoporosis, and several other major illnesses, how do we best present the data so that women can make informed choices? And how do we view this from a public health perspective?
Several issues pertain to the application of data such as these to clinical practice. Glasziou and Irwig (91) suggested that one needs to assess the underlying risk and potential benefits of any clinical therapy as it is applied to the individual patient. This would require sound estimates of the risk of heart disease and osteoporosis, to begin with, as well as a risk assessment for breast cancer. To this formula, I believe we must add the individual’ss fear or concern about the diseases considered, as is recommended for broader risk-benefit or cost-effectiveness considerations where “ quality-adjusted” life-years are used as an outcome (92). As shown in , age-specific mortality from cancer exceeds that from heart disease through age 70 years among women in the United States. The age-adjusted mortality from cancer has been higher than that from heart disease among women since 1990 (93). In 1995, the age-adjusted mortality from heart disease was 104 deaths per 100 000 women; from cancer, it was 110 deaths per 100 000 women. Among white women, the difference was greater (heart disease, 95; cancer, 109). Furthermore, as a measure of the burden of cancer, it is noted that the average years of life lost per death is 11.8 for heart disease and 19.5 for breast cancer (94). Thus, cancer has a major impact on women, and breast cancer is the leading cause of cancer diagnosis.
To these national data on the burden of cancer we might add that the apparent absence of lifestyle practices that can be modified readily to reduce the risk of breast cancer further heightens fear of this cancer. Contrast this with heart disease, for which we have a wide range of behavioral or clinical options that can reduce risk, many with fewer potential side effects than the increase in the risk of breast cancer caused by the use of postmenopausal hormones. These options include the following: greater physical activity; avoidance of weight gain; taking aspirin, vitamin E, folate, or cholesterol-lowering drugs; and treatment of high blood pressure. For example, taking a daily multivitamin reduces the risk of heart disease by some 25% and has he added benefit of reducing the risk of colon cancer, without any evident adverse effects (95,96).
Fig. 1.
Fig. 1. Age-specific mortality from heart disease and cancer among women in the United States, 1995. Data from Health, United States, 1996–1997, National Center for Health Statistics, Rockville, MD.
Other health advantages of postmenopausal hormones relate to the prevention of osteoporosis. The burden of this condition is primarily among older women, placing great demand for prevention strategies that do not increase the risk of breast cancer, which has a higher incidence than osteoporotic fractures prior to age 70. Growing evidence suggests that selective estrogen receptor modulators may offer protection against osteoporotic fractures by increasing bone density (97). It is likely that these agents will also offer substantial protection against breast cancer (reported at the National Osteoporosis Foundation meeting in Washington, DC, in June 1997). Given the causal relationship between estrogen levels and breast cancer, we must rapidly assess the risk and benefit profiles of these newer agents and develop a knowledge base sufficient to counsel women who are considering the many options for relief of menopausal symptoms and for long-term prevention of chronic disease. From a public health perspective, we must consider the underlying distribution of risk factors for other chronic conditions. The proportion of breast cancers and osteoporotic hip fractures in a population of hormone users will depend on the age distribution of that population. Historic trends indicate that heart disease has declined among women and men during the past 20 years (98). Hence, the trade-off of chronic conditions is not static but rather reflects a secular trend whereby cancer deaths now exceed heart disease deaths among white women and will do so among black women in the near future if current trends continue. On the basis of the follow-up of women in the Nurses’s Health Study whose reproductive histories, age at menarche, parity, and age at menopause reflect those of their birth cohort, even long-term use of postmenopausal hormones looks beneficial from the population perspective (99). Women who used postmenopausal hormones for 10 or more years had a 20% reduction in mortality that was statistically significant. This benefit of hormones was most pronounced among women who were at higher risk for cardiovascular disease. However, to achieve a reduction in mortality, some women within the population will pay the price of a breast cancer that they would not otherwise have developed. That is to say, some individuals experience a disadvantage as a result of an attempt to improve the overall health of the population. Thus, the action that will benefit public health and the individual’s best choice may differ. Further work is needed to improve our understanding and communication of these considerations.
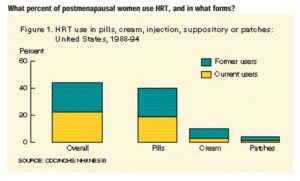
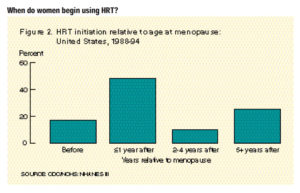
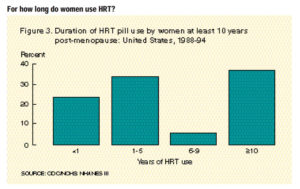
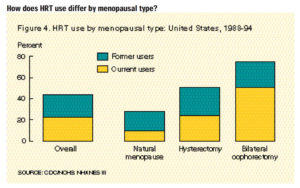
 Hormone replacement therapy (HRT) has been a staple treatment for menopausal symptoms for years. What many people do not understand is there is always some risk when we introduce hormones into the body, especially after menopause. The average age for menopause in women is between ages 45-55 years old, but age of onset does vary person to person. Through this period, women can experience disruptive and frustrating symptoms and HRT has been used for many years to help manage those menopausal symptoms.
Hormone replacement therapy (HRT) has been a staple treatment for menopausal symptoms for years. What many people do not understand is there is always some risk when we introduce hormones into the body, especially after menopause. The average age for menopause in women is between ages 45-55 years old, but age of onset does vary person to person. Through this period, women can experience disruptive and frustrating symptoms and HRT has been used for many years to help manage those menopausal symptoms.